Zhuzhou Weilai New Materials Techonology Co., Ltd
Tel:0731-22160654
Mobile:
+8615773363955(Mr. Duan)
+8615200507438(Ms.Zhang)
E-mail:sales@rheniumcn.com
Fax:0731-22160654
Address:No.103, Building 2, Tiantai
Jingu Industry Park, Tian Yuan
District,Zhuzhou,Hunan,China
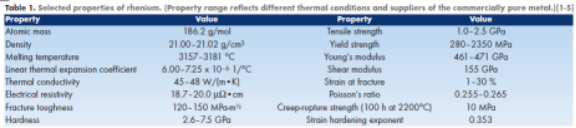
The element rhenium (Re) is a refractory metal that has gained significant recognition as a high-performance engineering material because it exhibits an exclusive combination of properties. Rhenium has the second highest melting point of all metals (after tungsten(W)), the third highest Young’s modulus (after iridium (Ir) and osmium (Os)), and the fourth highest density (after Os, Ir and platinum (Pt)). It also has one of the highest strain hardening exponents of all elements, a low coefficient of friction,and a high hardness, all of which result in excellent wear properties. Compared to the other refractory metals, Re has superior tensile strength and creeprupture
strength over a wide temperature range (up to approximately 2000°C). For example, between room temperature and 1200°C, its strength is approximately twice that of W. In addition, the rupture strength of rhenium is greater than that of W at temperatures as high as 2800°C. At 2500°C its strength is comparable to that of carbon composites. These properties imply that structures made of Re have excellent mechanical stability and rigidity, and they enable the design of parts with thin sections. It can also be concluded that Re is extremely attractive for high-temperature structural and energy system applications. A summary of properties is provided in Table 1.
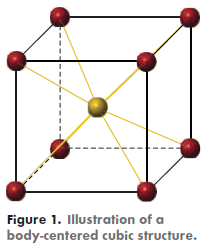
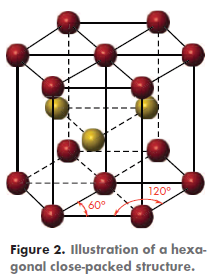
Interestingly, while the other refractory metals have a body-centered cubic (BCC) structure (Figure 1), Re has a hexagonal close-packed (HCP) structure (Figure 2). Consequently, it does not possess a ductile-to-brittle transition and, therefore, can safely be used at subzero temperatures.
Rhenium is the only refractory metal that does not form carbides. Even so, the solubility of carbon in Re is relatively high along with the wettability between these two elements. This combination of properties yields excellent bond strength between these two elements, and indeed,Re is used in contact with graphite and carbon composites, for example in high-temperature rocket engines and hot gas valves.
Rhenium is resistant to a wide range of harsh environments. It is highly resistant to corrosion in hydrochloric and sulfuric acids as well as in seawater. It also has low permeability to hydrogen and resists degradation in hydrogen and inert atmospheres at elevated temperatures. Re is immune to most combustion gases, with the exception of oxygen. On the other hand, Re is readily dissolved in nitric acid and other oxidizing acids. Moist air above 600°C also leaves Re vulnerable to oxidation due to the formation of Re2O7. Hence, oxidation protection is often sought through the development of Re alloys and the application of an oxidation-resistant top coating of Ir, Pt, or rhodium (Rh).
From:Properties and Applications of Rhenium and Its Alloys
Address:
No.103, Building 2, Tiantai Jingu Industry Park,
Tian Yuan District,Zhuzhou, Hunan, China.